Answer:
The percent yield of oxygen in this chemical reaction is 73.71 %.
Step-by-step explanation:
Experimental yield of oxygen gas = 115.0 g
Theoretical yield:
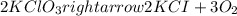
Mass of potassium chlorate = 400.0 g
Moles of potassium chlorate =
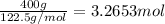
According to reaction, 2 moles of potassium chlorate gives 3 moles of oxygen gas.
Then 3.2653 mol of potassium chlorate will give:
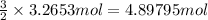
Mass of oxygen gas :
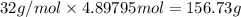
Percentage yield:
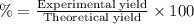
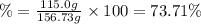
The percent yield of oxygen in this chemical reaction is 73.71 %.