Answer:
The mass of 22.05 mol of octane is 2,518 g.
Step-by-step explanation:
Molar mass of octane = 114.22 g/mol
Mass of 1 mole of octane is 112.22 g/mol.
Mass of compound:
Moles of compound × Molar mass of compound
Mass of 22.05 moles of octane is:
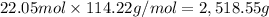
The closest answer from the given options is 2,518 g.