Answer: 0.125 grams
Step-by-step explanation:
Radioactive decay follows first order kinetics.
Half-life of give isotope = 20 minutes

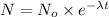
N = amount left after time t= ?
 = initial amount = 1.0 g
= initial amount = 1.0 g
 = rate constant
= rate constant
t= time = 1 hour = 60 min

N=0.125g
Thus amount left after 1 hour is 0.125 grams