Answer: Option (1) is the correct answer.
Step-by-step explanation:
A redox reaction is a reaction in which both oxidation and reduction takes place.
For example,
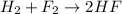
Oxidation-half reaction :
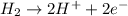
Reduction-half reaction :

Hence, we can see that electrons are involved in a redox reaction.
Therefore, we can conclude that in a redox reaction, electrons are the particles which are lost and gained in equal numbers.