Hello!
Determine the mass of 5.20 moles of C6H12 (gram-formula mass = 84.2 grams/mole).
We have the following data:
m (mass) = ?
n (number of moles) = 5.20 moles
MM (Molar mass of C6H12) ≈ 84.2 g/mol
Now, let's find the mass, knowing that:

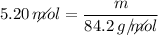
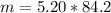

_______________________
I Hope this helps, greetings ... Dexteright02! =)