Answer:
1. the absolute pressure in the tank before filling = 217 kPa
2. the absolute pressure in the tank after filling = 312 kPa
3. the ratio of the mass after filling M2 to that before filling M1 = 1.44
The correct relation is option c (
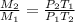 )
)
Step-by-step explanation:
To find -
1. What is the absolute pressure in the tank before filling?
2. What is the absolute pressure in the tank after filling?
3. What is the ratio of the mass after filling M2 to that before filling M1 for this situation?
As we know that ,
Absolute pressure = Atmospheric pressure + Gage pressure
So,
Before filling the tank :
Given - Atmospheric pressure = 100 kPa , Gage pressure = 117 kPa
⇒Absolute pressure ( p1 ) = 100 + 117 = 217 kPa
Now,
After filling the tank :
Given - Atmospheric pressure = 100 kPa , Gage pressure = 212 kPa
⇒Absolute pressure (p2) = 100 + 212= 312 kPa
Now,
As given, volume is the same before and after filling,
i.e.
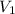 =
=
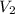
As we know that, P ∝ M
⇒
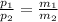
⇒
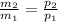
⇒
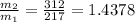 ≈ 1.44
≈ 1.44
Now, as we know that PV = nRT
As V is constant
⇒ P ∝ MT
⇒
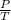 ∝ M
∝ M
⇒
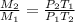
So, The correct relation is c option.