Answer:
87% bromine and 13% magnesium
Step-by-step explanation:
The compound contained 32.0 grams of bromine and 4.9 grams of magnesium.
We need to find its percent composition.
Mass of the sample = mass of bromine + mass of magnesium
= 32 g + 4.9 g
= 36.9 g
Percentage composition of bromine,
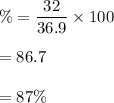
The percentage composition of magnesium,
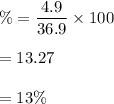
So, its percent composition is 87% bromine and 13% magnesium.