Answer :
(1) The electron configuration for the chloride ion will be,
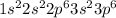
(2) The percent by mass of hydrogen in aspirin,
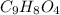 is, 4.44%
is, 4.44%
Explanation for Part 1 :
Element = chlorine
Atomic number = 17
As we know that the number of electrons is equal to the atomic number.
So, the number of electrons = 17
An electronic configuration of neutral chlorine element is,
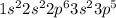
The chloride ion formed by the addition of an electron in chlorine element.
So, the electron configuration for the chloride ion will be,
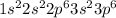
Explanation for Part 2 :
Mass of carbon = 12 g/mole
Mass of hydrogen = 1 g/mole
Mass of oxygen = 16 g/mole
The given compound is aspirin,
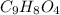
The molar mass of aspirin,
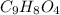 = 9(12) + 8(1) + 4(16) = 180 g/mole
= 9(12) + 8(1) + 4(16) = 180 g/mole
In the given compound, there are 9 atoms of carbon, 8 atoms of hydrogen and 4 atoms of oxygen.
The mass of hydrogen in aspirin = 8 g/mole
Now we have to calculate the percent mass of hydrogen in aspirin.
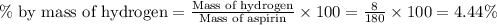
Therefore, the percent by mass of hydrogen in aspirin,
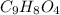 is, 4.44%
is, 4.44%