Answer: The mass of water present in the jar is 297.7 grams.
Step-by-step explanation:
We are given:
Mass of barium nitrate sample = 27 g
Solubility is defined as the maximum amount of solute that can be dissolved in a solvent at equilibrium.
The solubility of barium nitrate at 20°C is 9.02 g per 100 g of water
To calculate the mass of solution in which given amount of barium nitrate is dissolved, we apply unitary method:
9.02 g of barium nitrate is dissolved in 100 g of water
So, 27 g of barium nitrate will be dissolved in
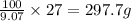 of water
of water
Hence, the mass of water present in the jar is 297.7 grams.