Answer:
The most stable ion is
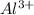
Step-by-step explanation:
The aluminium belongs to Group 13 of the periodic table. The elements of this group have 3 electrons of valence and due to the metalic characterictics the aluminuim has, it trends to lose those electrons to achieve stability.
Electrons are negatively charged (-1 each one), so when the neutral atom loses 3 of them it remains positively charged by +3