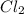Answer: The correct answer is
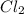
Step-by-step explanation:
Diatomic molecule is defined as the molecule in which only two atoms are present. The atoms present can be of different element or of the same element.
From the given options:
Option 1:

In this molecule, 3 atoms are present. One of Oxygen and two of hydrogen. Thus, it is a triatomic molecule, not a diatomic molecule.
Option 2:
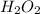
In this molecule, 4 atoms are present. Two of oxygen and two of hydrogen. Thus, it is a polyatomic molecule, not a diatomic molecule.
Option 3:
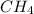
In this molecule, 5 atoms are present. One of carbon and four of hydrogen. Thus, it is a polyatomic molecule, not a diatomic molecule.
Option 4:
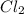
In this molecule, 2 atoms are present of chlorine element. Thus, it is a diatomic molecule.
Hence, the correct answer is