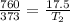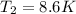Answer: The temperature required to boil will be 8.6 K
Explanation: The normal boiling point of water is taken as 100 °C
The pressure conditions at this temperature is 1 atm
Now, to calculate the temperature required to boil water present at 17.5 torr, we use Gay-Lussac's Law, which states that the pressure is directly proportional to the temperature at constant volume and number of moles.
Mathematically,
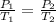
Initial conditions:
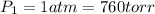
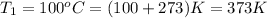
Final conditions:
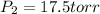
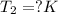
Putting in above equation, we get