Answer: The equilibrium will shifts to the left.
Explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
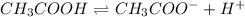
Thus if
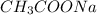 is added it will dissociate to give
is added it will dissociate to give
 and
and
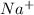

Thus as the concentration of
 increases, the equilibrium will shift in a direction where concentration of
increases, the equilibrium will shift in a direction where concentration of
 decrease i.e. the dissociation of
decrease i.e. the dissociation of
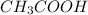 will be further depressed i.e the equilibrium will shift to the left.
will be further depressed i.e the equilibrium will shift to the left.