Answer: Option (b) is the correct answer.
Step-by-step explanation:
When an atom has 1 to 3 valence electrons then it shows that there are excess of electrons present in the outermost shell.
Therefore, in order to attain stability the atom will donate electrons to an electron deficient atom.
For example, valency of aluminium is 3 hence it forms
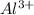 ions by donating electrons.
ions by donating electrons.
Thus, we can conclude that if an atom has 1 to 3 valence electrons then it will donate electrons.