A chemical equation that lists those species only which are participating in the reaction is said to be a net-ionic equation. It is commonly used in double-displacement reactions, acid-base neutralization reactions, and redox reactions.
In order to write the net ionic equation:
- The molecular equation is written first.
- The strong electrolytes are break into their respective ions with correct formula and number of ions.
- The common ions on both the sides that is on reactant and product side are cancelled out.
- The resulting equation is a net-ionic equation
The molecular equation between perchloric acid,
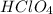 in water,
in water,
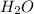 is:
is:
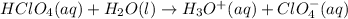
Breaking the strong electrolytes in their respective ions:
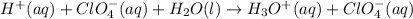
Cancelling the common ion,
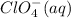 from both the sides:
from both the sides:
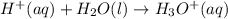
Hence, the net ionic equation between perchloric acid,
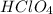 in water,
in water,
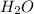 is:
is:
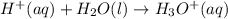
Since, the production of
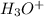 takes place so, the perchloric acid,
takes place so, the perchloric acid,
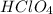 acts as an acid by donating proton,
acts as an acid by donating proton,
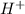 ion to water.
ion to water.