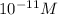Answer: Thus hydroxide ion concentration is
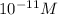
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
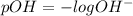
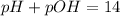
Given: pH=3
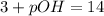
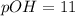
![11=-log[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/3k9bxhah5dfjkrnmz7fa1sb6nzx9me4zvw.png)
![[OH^-]=10^(-11)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/rbpmopvmmyifnlfyywcnun6gbiav3wg0wq.png)
Thus hydroxide ion concentration is