Answer:
23.30 liters of propane are required at STP to produce 75 g of water from this reaction.
Explanation:
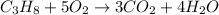
Water produced = 75 g
Moles of water =
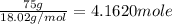
1 mole of propane gives 4 moles of water.
Then, 4.1620 moles of water will be obtained form:
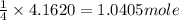 of propane
of propane
At STP ,1 mole of gas occupies = 22.4 L
Then, 1.0405 mole will occupy:
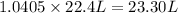
23.30 liters of propane are required at STP to produce 75 g of water from this reaction.