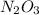Answer:
Step-by-step explanation:
Hi, the empirical formula is the minimal expression of the molecule composition and doesn't always represent the molecule in the reality.
On the other hand the molecular formula is a multiple of the empirical and represents how the molecule is composed in reality.
In this case they are the same.
Now, nitrogen has an atomic weight of 14 g/mol and oxygen one of 16 g/mol:
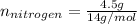
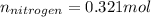
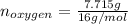
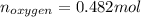
So the formula would be:
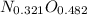
Dividing per 0.321:
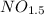
In natural numbers: