Answer: The equation is written below.
Step-by-step explanation:
Copper is the 29th element of the periodic table having electronic configuration of
![[Ar]3d^(10)4s^1](https://img.qammunity.org/2018/formulas/chemistry/high-school/dusvpqlsfuwl6mi9euxtltrk0y9rt739cr.png)
When an atom looses an electron to form positive ion, it is called an oxidation reaction.
Copper will loose 1 electron to form +1 ion. The equation for the formation of copper (I) ion from neutral copper atom follows:
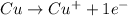
Hence, the equation is written above.