Answer:If the calorimeter decreases in temperature over the course of the reaction:
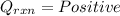
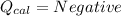 (heat is lost by the caloriemter)
(heat is lost by the caloriemter)
Step-by-step explanation:
Whenever reaction takes place in calorimeter tone out of two things can a happen:
1. If the temperature of calorimeter increases: During a reaction when heat is released in the calorimter. This heat released is then absorbed by the calorimeter (Law of Conservation of Energy) which results in increase in temperature of calorimeter.
Those reaction which add on heat to their surrounding are termed as Exothermic reaction.
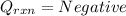
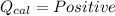 (Heat gained by the calorimeter)
(Heat gained by the calorimeter)
2. If the temperature of calorimeter decreases: During a reaction when heat is lost from the calorimter. This heat loss is due to the absorption of heat by reactants during the reaction (Law of Conservation of Energy) which results in decrease in temperature of calorimeter.
Those reaction which absorbs heat from their surrounding are termed as Endothermic reaction.
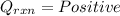
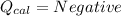 (heat is lost by the caloriemter)
(heat is lost by the caloriemter)