The original mass is m₀ = 1 μg.
The decay equation is
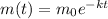
where
m = mass remaining after time t,
k = constant.
Let
t = t₁ = time for half-life.
Then
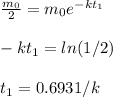
When t = 5 half-lives, then
t = 5*(0.6931/k) = 3.4657/k
The mass remaining is
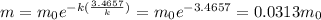
The mass remaining after 5 half-lives is
0.0313 μg
Answer: 0.0313 μg