Answer: 3808 ml
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
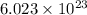 of particles.
of particles.
Standard condition of temperature (STP) is 273 K and atmospheric pressure is 1 atm respectively.
To calculate the moles, we use the equation:
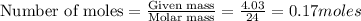
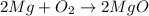
2 moles of magnesium react with= [tex[2\times 22.4=44.8L[/tex] of oxygen at STP
Thus 0.17 moles of magnesium react with=
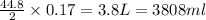
Thus the volume of oxygen required to react with 4.03 g of magnesium at STP is 3808 ml.