find how many half lives have passed
seems we are measuring in days
half life formula is as follows
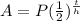
A=final amount
P=initial amount
t=time in a certain units (this time, it's days)
h=time of half life
so
we are given that P=1, A=0.6, t=3
find h
subsitute
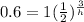
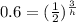
take ln of both sides
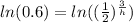
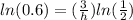
multiply both sides by h
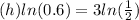
divide both sides by ln(0.6)
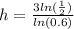
use your calculator
h≈4.07075
so about 4 days is the half life