Answer: Silver nitrate is the chemical reagent used to distinguish.
Step-by-step explanation:
Dissolve both compounds in water. And add silver nitrate to that solution.
1. In case of calcium chloride
The calcium chloride is soluble in water and thus forms ions in aqueous solution.The chloride ions present in the solution react with silver ions of silver nitrate to give white color precipitate of silver chloride.
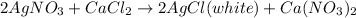
2. in case of calcium carbonate.
The calcium carbonate is poorly soluble in water. Since, it doesn't get dissolved in water.
When silver nitrate is added there will be no formation of any precipitate.