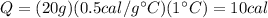Answer:
10 calories
Step-by-step explanation:
The thermal energy needed to increase the temperature of a substance is given by
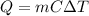
where
m is the mass of the substance
C is the specific heat of the substance
 is the increase in temperature
is the increase in temperature
In this problem, the mass of the ice is m=20 g, the specific heat is C=0.5 calories/gram°C, and the increase in temperature is
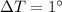 . Therefore, the energy required is
. Therefore, the energy required is