Answer:
Option B. 1 : 4.
Explanation:
In a lab 30% acid solution and 5% acid solution were mixed to prepare 10% acid solution.
We have to calculate the ratio of both the acids should be mixed to prepare 10% acid solution.
Let the volume of 30% solution was used = x ml
and the volume of 5% acid was used = y ml
Therefore the equation will form as
30%.x + 5%.y = 10%.(x + y)
0.30x + 0.05y = 0.10(x + y)
Now we transfer the similar terms in one side of the equation.
0.30x - 0.10x = 0.10y - 0.05y
0.20x = .05y
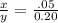
x : y :: 1 : 4
Therefore solutions will be mixed in a ratio of 1 : 4
Option B. 1 : 4 is the right answer.