Answer : The concentration of nitrate ions is 0.450 m.
Solution : Given,
Molarity of
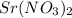 = 0.225 m
= 0.225 m
The balanced ionic equation is,
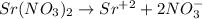
From the reaction, we conclude that the 1 mole of
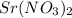 produces 2 moles of
produces 2 moles of
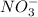 ions. That means the concentration of nitrate ions is twice the value of
ions. That means the concentration of nitrate ions is twice the value of
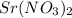 .
.
then, 0.225 m of
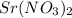 gives 2 × 0.225 of
gives 2 × 0.225 of
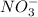 ions
ions
Now the concentration of
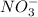 ions is equal to 0.450 m.
ions is equal to 0.450 m.