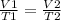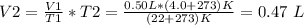Answer:
New volume of the balloon = 0.47 L
Step-by-step explanation:
Given:
Initial Temperature T1 = 22 C
Initial volume of the balloon, V1 = 0.50 L
Final temperature T2 = 4.0 C
To determine:
Final volume of the balloon, V2
Calculation:
Based on the ideal gas equation, pressure (P), volume (V) and temperature (T) are related as:
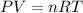
where n = number of moles of the gas
R = gas constant
Under conditions of constant P and n, the ideal gas equation becomes:
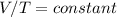
This is Charles Law which can also be expressed under a given set of initial and final conditions as: