Answer:
2.4358 grams of sodium chloride have 950.mg of sodium.
Step-by-step explanation:
Percentage of sodium in sodium chloride = 39.0 \%
Given mass of the sodium = 950 mg = 0.950 g
Let the mass of sodium chloride be M
Percentage of sodium:
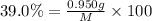
M = 2.4358 g
2.4358 grams of sodium chloride have 950.mg of sodium.