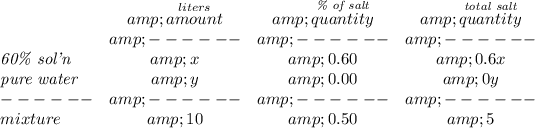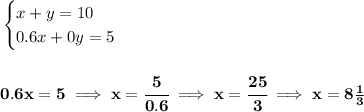so say, we add "x" of the 60% saline solution, now, how much salt is in that solution? now 60% is salt but the rest is something else, but only salt is 60% of "x", or (60/100) * x, or
0.6x.
likewise, for the 10 Liters, if only 50% is salt, then is just (50/100) * 10 or
5.
now... let's check the water whilst we add "y" amount, the water has no salt at all, freshwater for that matter, so, salinity is 0%. or (0/100) * y or
0.