Answer:
There are 0.004 moles in .499 grams of CaCO3
Step-by-step explanation:
Rememeber that to calculate the moles of a substance and its molar mass, in this example you are usin CaCO3 or calcium carbonate, which has a molar mass of 100.0869 g/mol that is a mol, so you just do a simple rule of three, where 100.0869 is 1 how much does .499 grams represent?:
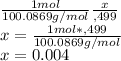
So there are 0.004 moles in .499 grams of CaCO3