Answer: The correct answer is total mass is unchanged.
Step-by-step explanation:
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
In a balanced chemical equation, total mass on the reactant side will always be equal to the total mass on the product side.
Every balanced chemical equation follows law of conservation of mass.
For Example: Formation of water from hydrogen and oxygen gas, the equation follows:
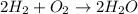
Total mass on reactant side:
![[2(1* 2)+(2* 16)]=36g/mol](https://img.qammunity.org/2018/formulas/physics/high-school/kjvh0xo5mv6iq9kqc4emkyjhaa58adbayf.png)
Total mass on product side:
![[2((1* 2)+16)]=36g/mol](https://img.qammunity.org/2018/formulas/physics/high-school/d935nifj62oqlr6gdvfvjppj0ddeooj1is.png)
As, mass on both the sides of the reaction remains the same.
Hence, the correct answer is total mass is unchanged.