Answer: The concentration of the stock solution will be 6.00 M.
Explanation: To calculate the molarity of the stock solution we use the formula:
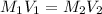
where,
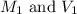 are the molarity and volume of the stock solution.
are the molarity and volume of the stock solution.
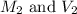 are the molarity and volume of the HCl solution.
are the molarity and volume of the HCl solution.
Given:
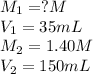
Putting values in above equation, we get:
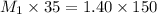
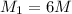
So, the concentration of the stock solution is 6M.