for half lives
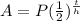
A=final amount
P=present amount
t=time in some units (this case it is days)
h=half life in days in th is case
so
given
we want 5% left
so A=5% of P or 5/100 of P or 1/20 of P or 0.05P
h=4.6
so
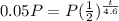
divide bth sides by P
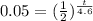
take ln of both sides

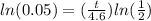
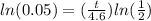
divide both sides by
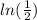
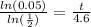
times both sides by 4.6
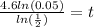
use your calculator
19.889t
so after about 20 days