Answer: Option (A) is the correct answer.
Step-by-step explanation:
For an equation to be completely balanced, there should be equal number of reactants and products.
In the given equation.
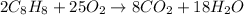
Number of carbon atoms on reactant side = 16
Number of hydrogen atoms on reactant side = 16
Number of oxygen atoms on reactant side = 50
Number of carbon atoms on product side = 8
Number of hydrogen atoms on product side = 36
Number of oxygen atoms on product side = 34
Hence, we can conclude that total number of reactants does not equal total number of products therefore, this equation is not completely balanced.