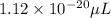Answer: The volume of single platinum atom in micro liters is
Step-by-step explanation:
We are given:
Volume of single platinum atom =
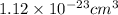
To convert this volume into micro liters, we use the conversion factor:
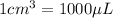
So,
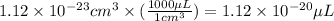
Hence, the volume of single platinum atom in micro liters is