Answer:
gftyrytyty
Step-by-step explanation:
According to Avogadro's number, in 1 mole of a substance there are
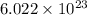 atoms and mass of copper is 63.55 g.
atoms and mass of copper is 63.55 g.
Hence, in
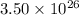 atoms the mass of copper will be calculated as follows.
atoms the mass of copper will be calculated as follows.
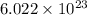 = 63.55 g
= 63.55 g
Mass in
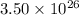 atoms =
atoms =
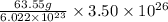
=
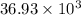
Thus, we can conclude that mass of copper is
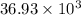 for
for
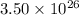 atoms.
atoms.