Answer : The number of moles of oxygen atoms are 135 moles.
Explanation : Given,
Moles of
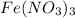 = 15.0 mole
= 15.0 mole
The given chemical formula is,
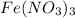
By the mole concept:
In
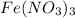 , there are 1 mole of iron (Fe) atom, 3 moles of nitrogen (N) atoms and 9 moles of oxygen (O) atoms.
, there are 1 mole of iron (Fe) atom, 3 moles of nitrogen (N) atoms and 9 moles of oxygen (O) atoms.
As, 1 mole of
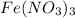 contains 9 moles of oxygen atoms
contains 9 moles of oxygen atoms
So, 15.0 moles of
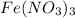 contains
contains
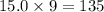 moles of oxygen atoms.
moles of oxygen atoms.
Hence, the number of moles of oxygen atoms are 135 moles.