Answer: The volume of water that must be added will be 1.417 L.
Step-by-step explanation:
Concentration of a substance is defined as mass of solute (in grams) present in the given volume of a solution (in L).
The equation representing concentration is given as:
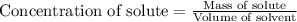
We are given:
Concentration = 3.81 g/L
Mass of sodium nitrate = 5.40 g
Putting values in above equation, we get:
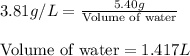
Hence, the volume of water that must be added will be 1.417 L.