Answer:
The percent yield is 58.12 %.
Step-by-step explanation:
Theoretical yield of aluminum oxide = 0.800 mol
Theoretical amount of aluminum oxide = 0.800 mol × 102 g/mol = 81.6 g
Actual or experimental yield of aluminum oxide = 0.465 mol
Experimental amount of aluminum oxide = 0.465 mol × 102 g/mol = 47.43 g
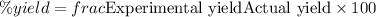
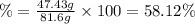
The percent yield is 58.12 %.