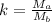Given:
Ca = 3Cb (1)
where
Ca = heat capacity of object A
Cb = heat capacity f object B
Also,
Ta = 2Tb (2)
where
Ta = initial temperature of object A
Tb = initial temperature of object B.
Let
Tf = final equilibrium temperature of both objects,
Ma = mass of object A,
Mb = mass of object B.
Assuming that all heat exchange occurs exclusively between the two objects, then energy balance requires that
Ma*Ca*(Ta - Tf) = Mb*Cb*(Tf - Tb) (3)
Substitute (1) and (2) into (3).
Ma*(3Cb)*(2Tb - Tf) = Mb*Cb*(Tf - Tb)
3(Ma/Mb)*(2Tb - Tf) = Tf - Tb
Define k = Ma/Mb, the ratio f the masses.
Then
3k(2Tb - Tf) = Tf - Tb
Tf(1+3k) = Tb(1+6k)
Tf = [(1+6k)/(1+3k)]*Tb
Answer:
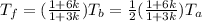
where