Answer: This reaction will produce a compound.
Step-by-step explanation:
Synthesis reaction is defined as the reaction in which two or more substances combine in their elemental state to produce a single product known as compound.
General chemical equation for this reaction follows:
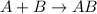
For Example: Formation of water from hydrogen and oxygen gas.
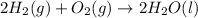
Hydrogen and oxygen gases are combining to produce a compound known as water molecule.
Thus, this reaction will always produce a compound.