For equilibrium reactions, it would be best to use the ICE method (Initial-Change-Equilibrium). Then, let x be the number of moles that reactedto form ICl.
2ICl ------> I2 + Cl2
I 0 0.75 0.75
C +2x -x -x
--------------------------------------------
E 2x 0.75 -x 0.75 -x
Kc = [I2][Cl2]/[ICl2]^2 = 0.11, the [I2] represents concentration of I2 in mol/L at equilibrium
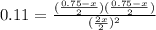
x = 0.451 moles
Thus [ICl] = (2x/2) = x =
0.451 mol/L