Answer:
75 mol
Step-by-step explanation:
The number of atoms in a mole of substance is 6.023X10²³ [Avagadro's number]
if the given sample of nickel contains 4.5X10²⁵ atoms it means the moles are
moles =
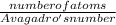
moles =
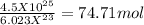
If we round of the given answer the number of moles = 75 moles