Step-by-step explanation:
A single displacement reaction is defined as the reaction in which only one element of a compound is displaced by another element.
For example,
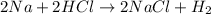
Therefore, when a metal reacts with an acid then hydrogen atom present in the acid is displaced by the metal atom.
As a result, a salt is formed along with evolution of hydrogen gas.
This reaction is similar to a single displacement type reaction.