Answer:
Option B, There are 48 neutrons within each isotope
Step-by-step explanation:
As we know ,
Mass number (M) is equal the sum of atomic number (A) and neutrons (n)
Mathematically, it can be represented as -
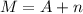
Given,
Atomic Mass (M)
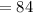
Atomic Number (M)
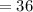
Substituting the given values in above equation, we get -
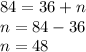
Option B is correct