Answer:
Volume= 2,854 liters
Step-by-step explanation:
In order to solve this you have to remember that the formula for the density is:
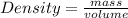
With this formula you just have to solve for volume in order to calculate what is the volume in lithers of 3,14kg of glycol, remember that the density of glycol is: 1,1g/ml^3
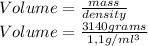
So the volume would be 2,854,54 ml, or 2,854 liters.