Answer:
Explanation: Law of Definite Proportions state that in a chemical compound, the constituent element will always exist in a fixed ratio which does not depend on the source of preparation.
So when hydrogen is burned in oxygen to form water, the composition of water formed does not depend on the oxygen but it depends on the hydrogen itself.
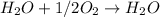
Thus the reaction usually depends on the limiting reagent ad hence , in the above reaction, hydrogen is the limiting reagent. Thus the reaction will depend on the hydrogen, not on oxygen.