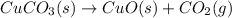Answer : The balanced chemical reaction will be:
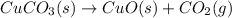
Explanation :
Balanced chemical equation : It is defined as the number of atoms pf individual elements present on reactant side must be equal to the product side.
In the balanced reaction, the reactants and products are separated by right arrow.
Reactants are the species that is present on the left side of right arrow and products are the species that is present of the right side of the right arrow.
As per question, when solid copper(ii) carbonate decomposes the is gives solid copper(ii) oxide and carbon dioxide gas as a products.
Decomposition reaction : It is a type of reaction in which the larger molecule decomposes to give two or more smaller molecules.
The balanced chemical reaction will be: