Answer:
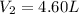
Step-by-step explanation:
Hello,
In this case, by means of the Boyle's law which allows us to understand a gas' volume-pressure relationship through an inversely proportional relationship:
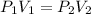
We solve for the final volume
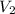 as required, including the conversion from mmHg to atm for the final pressure:
as required, including the conversion from mmHg to atm for the final pressure:
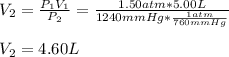
Regards.